Surface Tension
Surface Tension: Overview
This topic covers concepts such as Surface Tension, Intermolecular Forces, Molecular Theory of Surface Tension, Cohesive Force, Adhesive Force, Molecular Range of Intermolecular Forces, Force Diagram of Liquid Molecules, etc.
Important Questions on Surface Tension
The maximum area of tension reinforcement in beams shall not exceed:
Water rises to a height of in a capillary tube and mercury falls to a depth of in the same capillary tube. If the density of mercury is and its angle of contact is and density of water is and its angle of contact is , then the ratio of surface tensions of the two liquids is
The radii of two soap bubbles are and . In isothermal conditions, two meet together in vacuum. Then the radius of the resultant bubble is given by
A film of water is formed between two straight parallel wires of length each separated by . if their separation is increased by while still maintaining their parallelism, how much work will have to be done (surface tension of water )
Radius of a soap bubble is increased from to work done in this process in terms of surface tension is
Radius of a soap bubble is increased from to work done in this process in terms of surface tension is
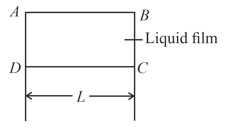
A liquid film is formed over a frame as shown in figure. Wire can slide without friction. The mass to be hung from to keep it in equilibrium is
There is horizontal film of soap solution. On it a thread is placed in the form of a loop. The film is pierced inside the loop and the thread becomes a circular loop of radius . If the surface tension of the loop be , then what will be the tension in the thread
A long wire is placed horizontally on the surface of water and is gently pulled up with a force of to keep the wire in equilibrium. The surface tension in of water is
A thin metal disc of radius floats on water surface and bends the surface downwards along the perimeter making an angle with vertical edge of disc. If the disc displaces a weight of water and surface tension of water is , then the weight of metal disc is
A wooden stick long is floating on the surface of water. The surface tension of water . By putting soap solution on one side of the stick, the surface tension is reduced to . The net force on the stick will be
Floating a needle on the surface of the _____ is an example of surface tension.
Explain some examples which illustrate the existence of surface tension.
Adding soap _____ the water's surface tension so the drop becomes weaker and breaks apart sooner.
Small insects such as the water strider can walk on water because their weight is not enough to penetrate the surface.
What are the examples illustrating the effects of surface tension?
Which one is the strongest intermolecular force?
How does soap decrease the surface tension of water. Explain in brief.
Impurities always decrease the surface tension of a liquid.
The surface of a liquid behaves like a stretched membrane. Explain in brief using a force diagram.
